They didn’t know of course, that I just finished Only a Theory last week, loved it, had no idea Miller was coming to speak, and was overjoyed to be informed of it. Needless to say, they were annoyed that the guy wasn’t in fact a creationist, and I wasn’t in fact pissed off. The talk was wonderful though. He is as good of a speaker as he is a writer. I think he fills a very needed role in the evolution/creationist debates—as a Christian himself, he is able to speak directly to fears that evolution and theism are fundamentally incompatible. As Miller points out, people’s beef with evolution is not really scientific, it’s philosophical—the fear that evolution takes away our ability to be moral human beings with purpose in our lives.
His talk was basically a summary of OAT, but for those who haven’t had a chance to read it yet, I’ll outline the talk for you here.
He started off with an overview of all the legal challenges to evolution that have been cropping up within only the last four years: there have been two federal trials (outlined below) and two state elections (OH and KS) that hinged on questions of evolution. Anti-evolution measures were passed in six state legislatures during this period, and 44 states had local measures passed in counties or in individual communities.
At the federal level, there was the 2004 case in Cobb County, Georgia, where the school board decided to put warning labels on the inside of the local high school biology textbook (written by Miller, incidentally). The stickers read, “This textbook contains material on evolution. Evolution is a theory, not a fact, regarding the origin of living things. This material should be approached with an open mind, studied carefully and critically considered." Miller pointed out that the scientific definition of “theory” is not “we don’t really know,” and really a theory is of a higher order than a fact, since theories explain sets of facts. Additionally, he protested that the sticker conveys a false sense of certainty about all other fields of biology, and implies that, for instance, ecology and cell biology should not “be approached with an open mind, studied carefully [or] critically considered.” Kind of insulting to all biologists, really.
And then of course there was the famous Dover trial in 2005, for which he served as an expert witness for the side of science. In this case, the local school board was guided by the Discovery Institute’s booklet, “Intelligent Design in Public School Science Curricula: A Legal Guidebook,” and bought two sets of the Intelligent Design textbooks “Of Pandas and People” for the school library, as recommended by the booklet. The board also demanded that the biology teachers re-write their curricula to include intelligent design. They refused. The school board pushed back, demanding then that the teachers at least use an intelligent design lesson plan that the school board wrote for them. They refused. The school board finally asked the teachers to just read a statement to the class about the “weaknesses” of evolutionary theory and the teachers once more refused, forcing the school superintendent to come in and read the statement to the classes while the teachers stood outside. There was immediate protest from the community, of course, and a first amendment lawsuit was quickly filed against the Dover Area School District.
A group of scientists, including Miller, volunteered to serve as expert witnesses. Miller noted in his talk that despite ID proponents’ eagerness to subpoena “evolutionists” for a courtroom trial and force them to speak under oath, 5 of 8 ID witness volunteers dropped out of the case (including William Dembski, who wrote the document linked to in this paragraph).
The details of the ruling and the evidence is really quite fascinating, and you can find a thorough summary at Wikipedia along with a link to the judge’s decision. (The judge ruled, by the way, on the side of evolution.)
Miller then went on to outline just some of the purported evidence for Intelligent Design. In his talk on Friday, he just went over one major piece of the ID case—the “irreducible complexity” of the bacterial flagellum, but his book has many more, all of which he carefully considers and thoroughly destroys.
Michael Behe, one of the major ID theorists, defines irreducible complexity basically as the inability to produce a complex structure piece by piece while maintaining one function. For example, you couldn’t evolve a mousetrap because a mousetrap with only a couple of its parts is no longer a mousetrap—all of the parts are necessary for its mouse-catching function. The IDers say that the bacterial flagellum is an example of such an irreducibly complex structure, as it would not be able to function as a flagellum without any of its parts.
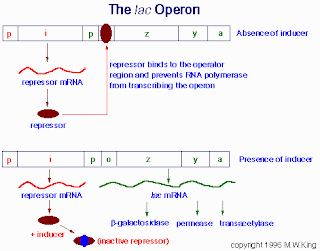
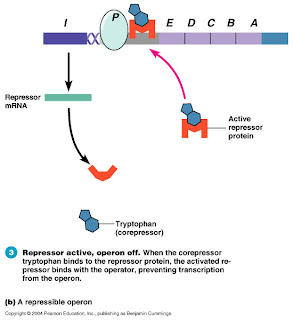
As Miller described in his talk and in OAT, the major problem with this argument is that it assumes that modern-day biological structures have evolved from earlier structures with similar functions. The fact is, as structures evolve, they often acquire radically new functions. (See my earlier post on the tryptophan operon for a biochemical example of this.) The same goes for the bacterial flagellum. It turns out that the base of the flagellum has incredible homology with another bacterial structure called a Type III Secretory System, used by bacteria to pump poisons. Additionally, other parts of the flagellum are made of a number of other proteins that exist elsewhere in the cell, performing other functions. So, unlike the predictions of the Irreducible Complexity argument, it is in fact possible to “take away” parts of a complex biological machine and have it still function—it’s just a different function.
At this point in the talk, Miller switched gears. He asked: well, if the best arguments the IDers can come up with don’t hold water, why is there such popular support for ID? He put a lot of emphasis on a quote from an NPR interview about evolution with former Sen. Rick Santorum, which I think is significant enough to reproduce here in its entirety:
"It has huge consequences for society. It's where we come from. Does man have a purpose? Is there a purpose for our lives? Or are we just simply the result of chance? If we are the result of chance, if we're simply a mistake of nature, then that puts a different moral demand on us. In fact, it doesn't put a moral demand on us."
Miller’s point was that Santorum--and approximately half of Americans--reject evolution because they see it as materialistic to a fault: denying souls, denying order, purpose, and meaning. Essentially, Miller argued, the design movement has forced science into a corner: out of opposition to the ID movement, scientists find themselves having to take the exact opposite position—that there is no design in the universe.
Miller argued the opposite: the universe has a design; it is the design of evolution. Basically, he said that evolution is an inherent, predictable property of life. It explores adaptive space in a predictable way, filling the same ecological niches with similar types of organisms. All organisms and biological structures do have a function, but it is a function that is driven by evolution. Miller went through several examples--such as the elegance of the genetic code, the specificity of proteins, the homology between different sorts of animals—-to illustrate this evolutionary “design.” He would probably note that the fact that I just put the word “design” in quotes in that last sentence is a sign that I am a scientist who has become afraid of the word because of its attachment to the anti-science ID movement. We, as the science-loving public, must reclaim that word, he argued. Regardless of our own beliefs about religion, we must show that the design of evolution does not mean our species and our lives are ruled only by chance and accident--we can still have purpose in our lives, and we are not "mistakes." Science’s derided materialism is its virtue, not its downfall, because it allows science to find natural explanations for natural phenomena. The ID movement was founded on an opposition to this materialism but Miller (like C.S. Lewis in the title of this post) argued that materialism shouldn’t frighten us, as a society, away from science. The scientific process cannot make claims about good and evil; this is society’s job as a whole. Science, and specifically evolution, Miller argued, will not make us immoral. It will only teach us about our place amongst the life on Earth, a place uniquely suited for us and our lives, each with its own unique purpose for the greater good.
Miller deftly answered the questions from the audience after his talk. This is obviously a man who has given many, many talks to diverse audiences, has heard every possible question, and has thought about all of them. His answers were eloquent and well-considered. If there were any IDers in the audience, they did not speak up in the Q&A period. As a friend and I discussed afterwards, they really wouldn’t have much to protest in the talk. Miller really covered all of the bases, and as a practicing Roman Catholic, could hardly be told that evolution is threatening to religion. If you haven’t yet read Only a Theory I suggest you pick it up at your local library. Not only does it thoroughly annihilate the ID position from every angle (useful reading for when you are forced to argue with a creationist) and discuss the larger anti-science implications of the anti-evolution movement, but it also provides a useful and much-needed perspective on the relationship between evolution and philosophical questions of morality and purpose. Read the book, and if you are lucky enough to see an announcement of a talk by Ken Miller, go and see him--and bring your friends.